Showroom
Pharmaceutical grade raw materials like PVA sponge, transparent PP, lint protected cellulose fiber and filter paper made General Ophthalmic Products are specially developed to fulfill various eye care, diagnosis and treatment requirements. These medical items are used to determine eye pressure, to examine tear releasing rate from eyes and for fitting of hard contact lens. Convenient to use, all these ophthalmic items are available in sterilized form to avoid contamination.
Premium quality Eye Care Products offered by us are used to take care of eyes during diagnosis, pre surgery and post surgical period. Raw materials like lint proof non woven fabric, soft tissue paper, PE film, Aloe vera extract and glycerol have been sued to prepare these ophthalmic care items. Accessible in sterilized form, all these products are non inflammatory and are effective in maintaining moisture level of eyes.
Non toxic PVC reinforced tube, DEHP plasticizer and silicon rubber made Disposable Cannula is used as a suitable nasal oxygen conveying medium for infants, children and adults. The gas delivery process of this transparent and recyclable cannula can be observed easily. Free from leakage, this item is available with flared or standard tip option. Light in weight, this double lumen type item is equipped with machine joint section and oxygen outlet.
Multi layered PP material and SSMMS type non woven fabric made Surgical Gowns are appreciated for their light weight nature and breathable quality. These disposable gowns have been sterilized via EO or gamma sterilization technique. Advanced ultrasonic welding method is used to treat their seam section. Homogeneous density, water proof design, accessibility in light blue color and smooth surface are some key attributes of these gowns.
Made of medical grade cotton leno weave fabric, viscose and paraffin, the Dressing Pads are used as hygienic wound affected body parts. Featured with high absorption capacity, these soft pads are of anti allergic quality. The anti bacterial properties of these pads prevent infection of injured or burnt body parts during cleaning. Available in sterilized form, these are useful to avoid cracking of skin of bruised body part.
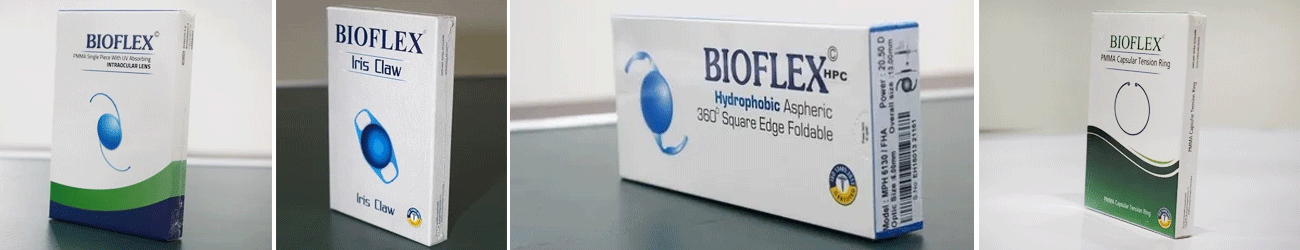
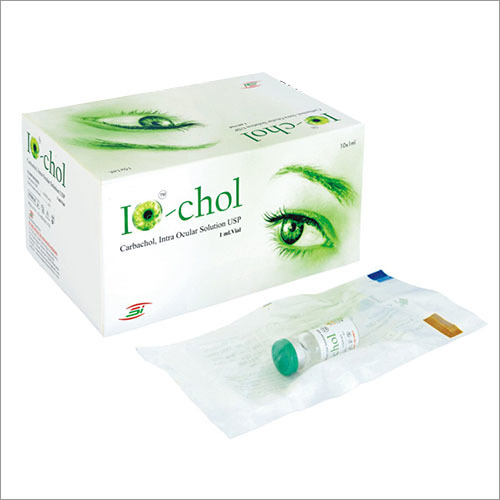
The specially formulated pharmaceutical solutions available as part of Corneal Products are used for treating various complications of eyes especially before or after surgical procedures. Standard grade raw materials like sodium hyaluronate gel and dextran have been utilized for their composition. These are suggested for patients who have gone through surgeries like corneal transplant. Besides improving vision, these medical products are effective in hydrating eyes and protecting ophthalmic tissues.
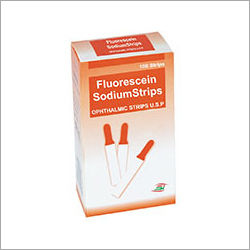
Specially designed Ophthalmic Paper is utilized to assess tear discharge level of eyes through Schirmer testing procedure. This paper is accessible in roll form with golden and silver color options. This paper is designed with multiple colors that are referred as rainbow colors. These colors are meant for determining ph content of discharged tear by touching its surface at the inner side of lower eyelids.
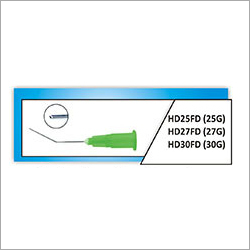
Standard quality stainless steel made ophthalmic Microsurgery Knives are perfect for dealing with soft and highly sensitive tissues of eyes during surgical procedures. Apart from their accurate cutting method, these knives are preferred for their hypo allergic quality and non hazardous quality. These knives are designed with easy to grip handle, angled tip and sharp blade. These disposable items are accessible in sterilized form.
Standard grade polypropylene, polyethylene, SMS non woven fabric and spun lace fabric made Surgical Disposable Sheet is widely used in hospitals and clinics for avoiding contamination or infection during surgeries. Accessible in white and light blue color options, this disposable sheet is preferred for its good air permeability level, biodegradable quality, water repellant nature, non toxic quality, soft and smooth surface.
Made of pure latex material, the offered Surgical Gloves are worn by surgeons during conducting surgeries for averting contamination. These gloves have been sterilized by using Gamma ray. The anatomical design of these gloves is meant for lowering hand fatigue during surgeries. Convenient to wear, the soft inner part of these gloves ensures optimum comfort to their users. Good elasticity level and recyclable nature are some key attributes of these products.
 |
SURGITECH INNOVATION
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |













 Send Inquiry
Send Inquiry Send SMS
Send SMS Call Me Free
Call Me Free

